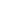
Clofarabine
It is a nucleotide analogue developed by Bioenvision and manufactured by Genzyme. On December 29, 2004, it was approved by FDA for the treatment of refractory or relapsed acute lymphoblastic leukemia (ALL) in children.
key word:
Category:
Product Description
Integrity, innovation, cooperation, sharing, win-win
The company integrates multiple resources and strives to build a large health industry finance platform company integrating pharmaceutical, intelligent medical, bio pharmaceutical R&D, industrial production and sales.
The chemical reactions involved in the existing products include Foucault reaction, nitration reaction, sulfonation reaction, hydrogenation reaction, fluorination reaction, chlorination reaction, bromination reaction, diazotization reaction, format reaction, etc.
It is a nucleotide analogue developed by Bioenvision and manufactured by Genzyme. On December 29, 2004, it was approved by FDA for the treatment of refractory or relapsed acute lymphoblastic leukemia (ALL) in children.
Clofarabine is the first new drug approved for pediatric ALL in the last 10 years. It can bring continuous remission to some children and create conditions for some children to receive bone marrow transplantation. It is a new effective and well-tolerated treatment option for children with high resistance leukemia. Clofarabine has also shown promise in children with relapsed or refractory acute myeloid leukemia.
Clofarabine HAS POTENTIAL BROAD SPECTRUM ANTITUMOR PROPERTIES: With more than 20 clinical indications in the United States, CLOfarabine is a broad spectrum anticancer drug with great potential.
Now in clinical indications are: breast, lung, colorectal, prostate, kidney cancer, cervical cancer, pancreatic cancer, skin cancer, bladder cancer, non-small cell lung cancer, oral cancer, nasopharyngeal carcinoma, laryngeal cancer, maxillary sinus carcinoma, esophageal cancer, uterus tumor, melanoma, leiomyosarcoma of the bulk of the phase I clinical study has been done. Acute myelogenous leukemia, chronic lymphoma, and non-Hodgkin's lymphoma are in phase II clinical studies, and inhibition of graft rejection is in phase I clinical studies. So while it is used to treat acute leukemia, its potential indications include many solid tumors as well as some immune diseases.
Related products
Sitagliptin Phosphate Monohydrate
Upadacitinib(Scientific research)
Product Consulting

Follow WeChat
Anhui Union Pharmaceuticals Technology Co., Ltd.
Service line:
+86-21-54285906
E-mail:2880705932@qq.com
Add:4th Floor, Building B3, Creative Industry Park, Hefei, Anhui P. R. China.
Copyright © 2022 Anhui Union Pharmaceuticals Technology Co., Ltd.